1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts


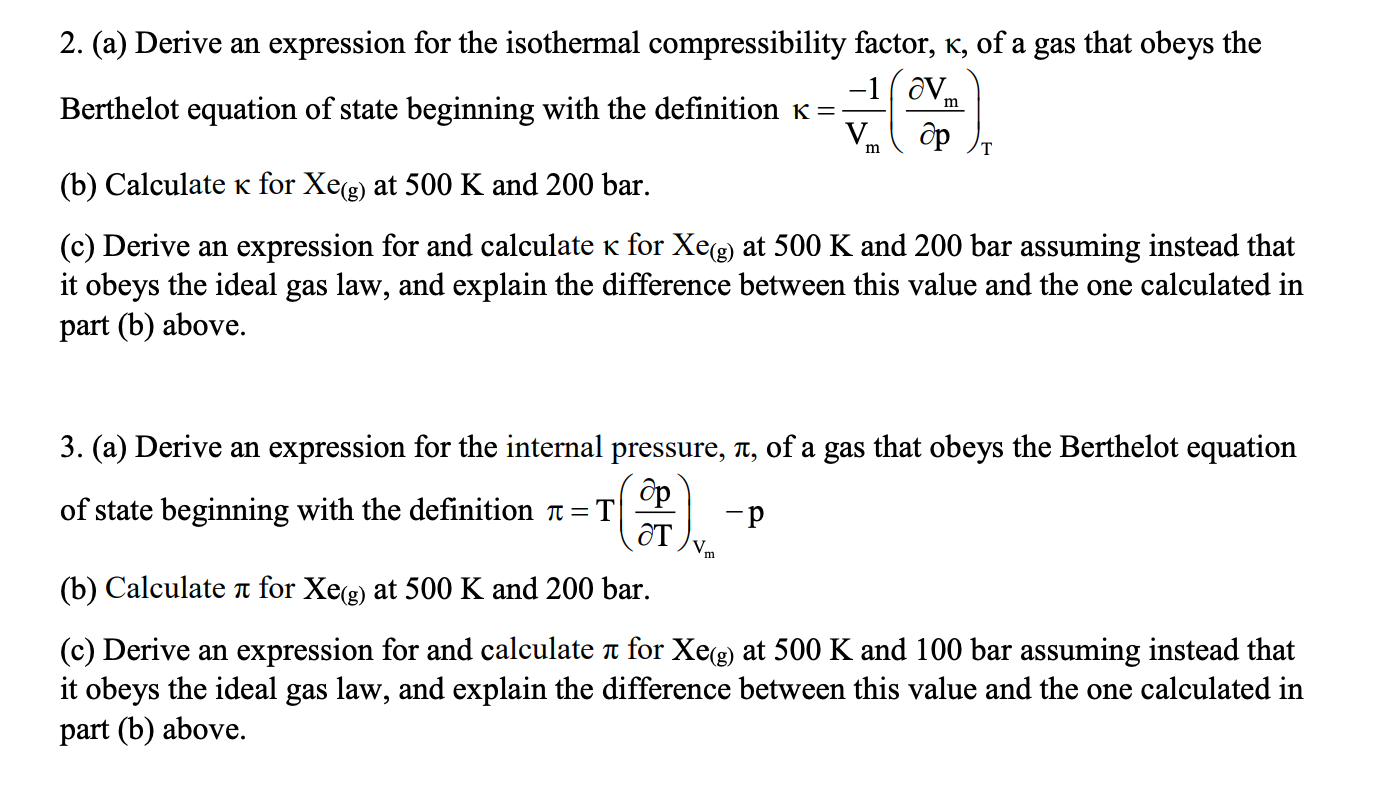
The Boyle Temperature in a two-term virial equation of state

Van der Waals Equation Practice Problems

The Boyle temperature of a van der Waal gas is `-246^(@)C`. Its critical temperature on absolute
Chapter 11.1: Real Gases - Chemistry LibreTexts
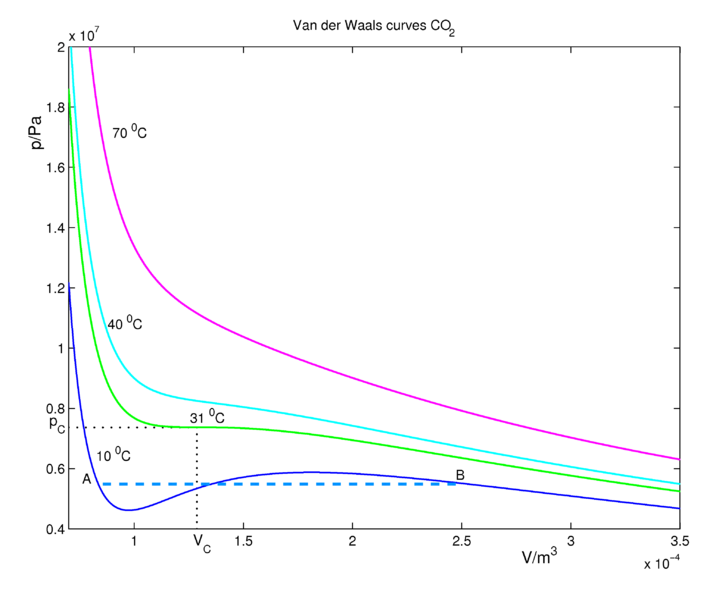
16.3: A Cubic Equation of State - Chemistry LibreTexts
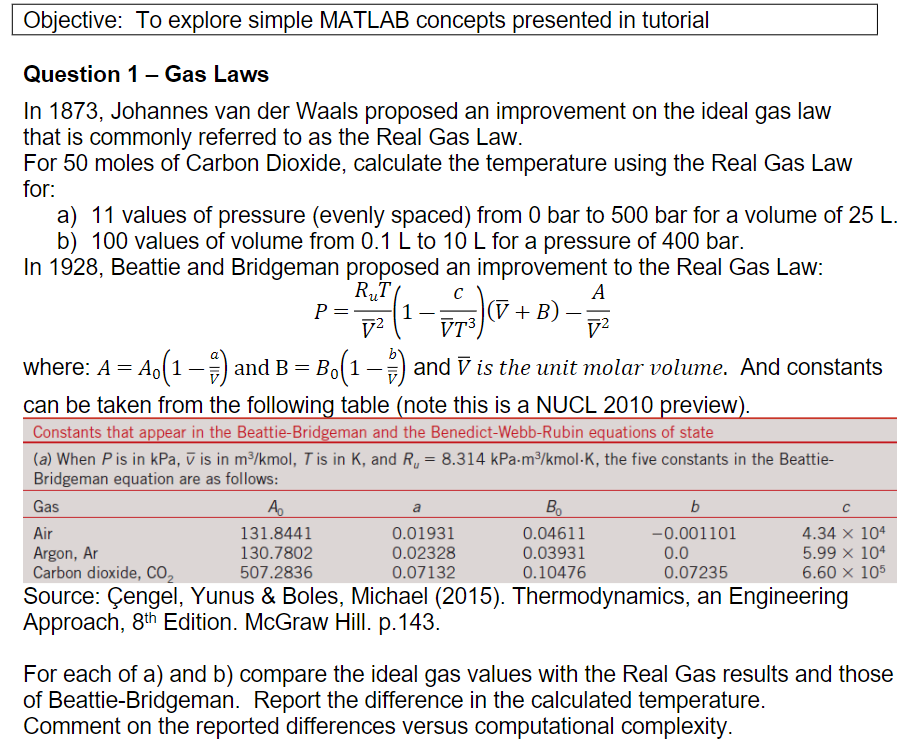
Question 1 - Gas Laws In 1873, Johannes van der Waals
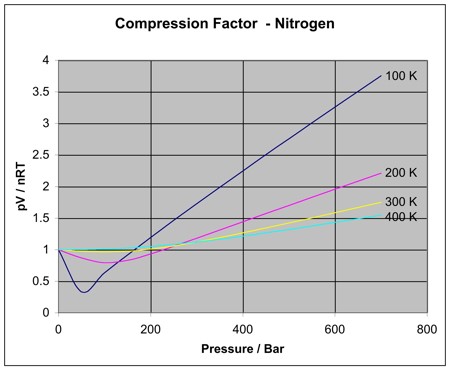
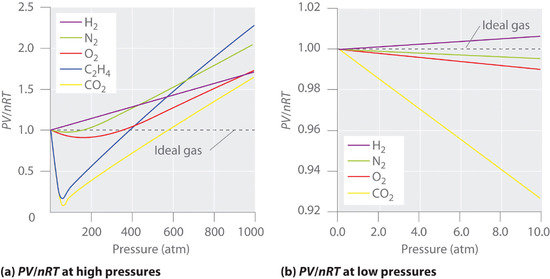
1.7: Real Gases - Chemistry LibreTexts
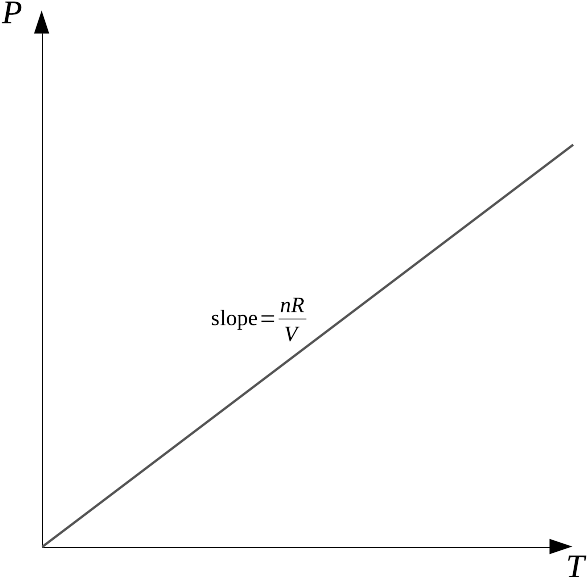
1.8: The ideal gas law, functions and derivatives - Chemistry LibreTexts
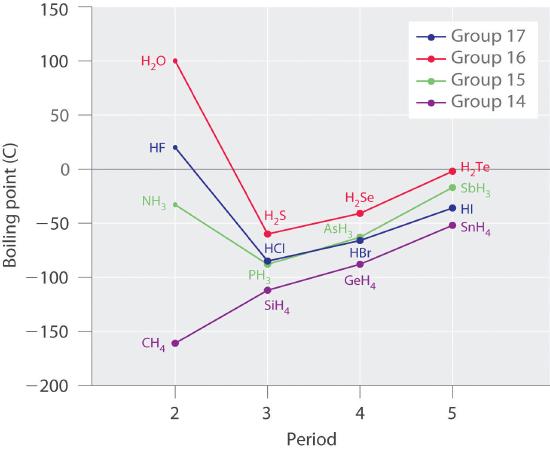
11.2: Intermolecular forces - Chemistry LibreTexts
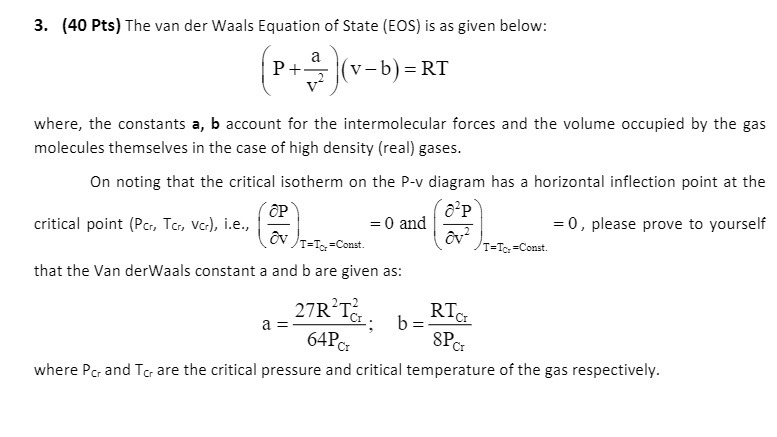
SOLVED: Please prove to yourself that the Van der Waals constants a and b are given as: 3. (40 Pts) The van der Waals Equation of State (EOS) is as given below: (
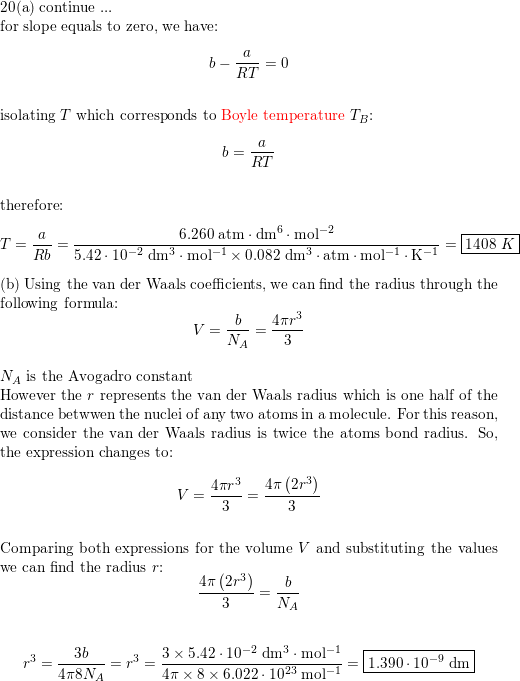
molecule regarded as a sphere. (b) Use the van der Waals parameters for hydrogen sulfide of the Resource section to calculate approximate values of (i) the Boyle temperature of the gas and (ii) the radius of a

For a van der Waal's gas, determine Boyle Temperature. [ given mathrm{a}=4.5 mathrm{atm} mathrm{L}^2mathrm{mol}^{-2}, mathrm{b}=0.9 mathrm{L} mathrm{mol}^{-1} and R=0.082 mathrm{L} mathrm{atm} mathrm{K}^{-1} mathrm{mol}^{-1}].609.8K6.09K273K60.98K
:max_bytes(150000):strip_icc()/GettyImages-1864388765-2eb7d042828e47518204ac93b75e5445.jpg)