Five Common Mistakes Submitting a Premarket Notification

How you can avoid the most common errors made when submitting a 510(k), the “premarket notification,” with simple measures

Industry opening up to idea of pre-market notification process for supplements

Obtaining FDA 510k Clearance for Class II Medical Devices

Mock FDA 510(k) Filing

Premarket Notification The 510(k) Process

5 Tips for Successful Medical Device Registration Across Global Markets
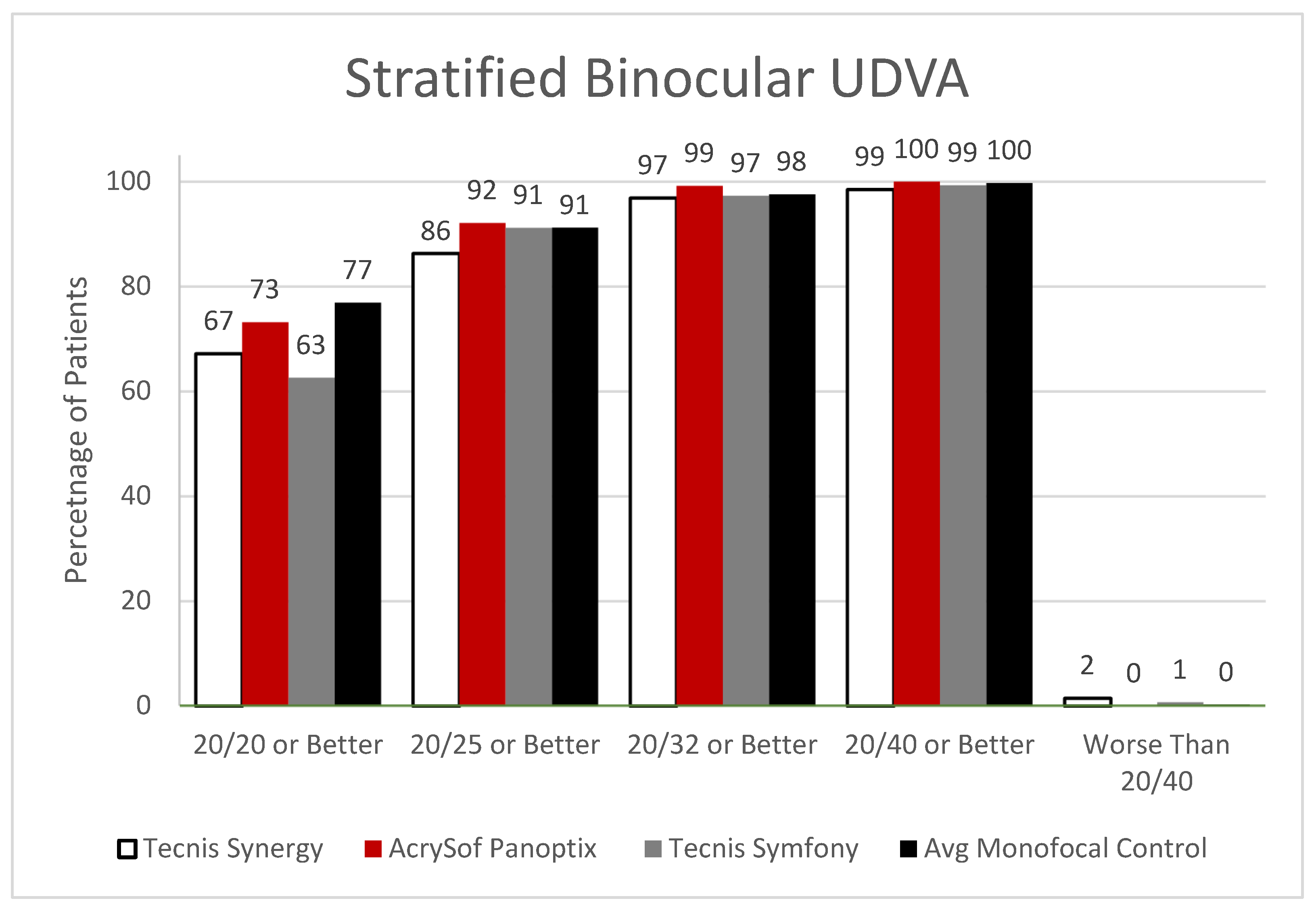
JCM, Free Full-Text
Cancel, Deactivate, or Reactivate a Facility Registration

Medical Device Academy Blog Archive

510(k) Pre-Market Notification Project

PPT - Premarket Processes & Pathways to Market Pre-amendment, Exempt, 510(k), and 513(g) PowerPoint Presentation - ID:1605402









